Stockton’s Atomic Force Microscope Helps Chemists Get a Closer Look at Semiconductor Fabrication
Anthony Bautista, a senior Chemistry major, Daniel Do, a freshman Biochemistry Molecular Biology major, Jason Shulman, associate professor of Physics, Mike Maffucci, a freshman Chemistry major, and Wooseok Ki, assistant professor of Chemistry, collaborate in the lab to fabricate semiconductors that require only sunlight and water to produce hydrogen as a renewable energy source. Not pictured is Anastasia Morozova, a junior Chemistry major, who is also working on the project.
Stockton University researchers are developing semiconductors that require only sunlight and water to produce hydrogen as a renewable energy source.
But the materials that Assistant Professor of Chemistry Wooseok Ki and his students are working with are invisible to the naked eye.
This obstacle was overcome with help from Jason Shulman, associate professor of Physics, who employed Stockton’s new Atomic Force Microscope to get a closer look.
Before the semiconductors can be made, a template of 500-nanometer polystyrene (plastic) microspheres must be tightly packed on a substrate to serve as a foundational structure. They are too small to work with by hand.
“It’s hard to convey their scale. Five hundred nanometers is about the wavelength of green light. A line of 100 spheres placed side by side will be about the thickness of a piece of paper,” explained Shulman.
Ki and his students developed a simple technique where the microspheres are dispersed and self-assemble into the proper configuration. But, in order to know if their technique works, they need to see the results. Stockton’s Atomic Force Microscope helps the team see their work.
Imagery of the self-packed spheres captured by the Atomic Force Microscope reveal a golden-colored honeycomb pattern. But the spheres aren’t truly golden. The false color was rendered by the microscope to enhance the visualization.
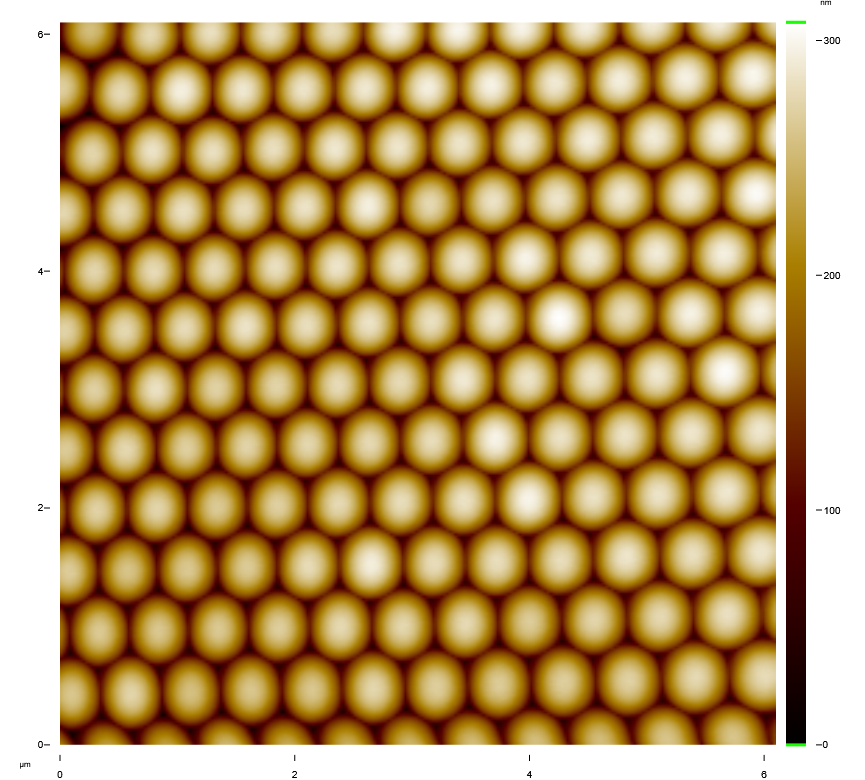
The images confirmed that the self-assembly technique produces perfectly packed spheres for the semiconductor template.
Ki is grateful for the interdisciplinary collaboration that offers insights where visual observation falls short.
The four undergraduate students working on the project said they are gaining invaluable experience in the fields they hope to pursue.
Anthony Bautista, a senior Chemistry major, hopes to work in the renewable energy field as a research and development scientist.
“Currently, this is one of our biggest breakthroughs in our research,” he said. “Our team has been trying to accomplish the task of developing a monolayer since last summer and to know that we finally found a method that works, is exciting.”
The next step in the fabrication involves pouring the semiconductor solution onto the template of closely packed microspheres arranged in a monolayer. A heat treatment then evaporates the polystyrene spheres, leaving pores, which completes the fabrication of the porous semiconductor.
Ki and his students are working with Rutgers University researchers to characterize the performance of the porous semiconductors, which releases their end goal of hydrogen gas. With scientific instrumentation at Rutgers, the team will be able to precisely quantify the amount of hydrogen produced by the semiconductors.
Anastasia Morozova, a junior Chemistry major on the research team, hopes to become a professor to “keep doing exactly what I am doing now, renewable energy and energy storage research.”
The trial-and-error process is sharpening her skills as a problem solver.
“There's no lab manual to tell you step-by-step how to make the best semiconductor,” she said. “Many graduate students find that daunting, but I'm already quite used to it.”
To learn more about the programs offered within Stockton’s School of Natural Sciences and Mathematics, visit www.stockton.edu/nams.
Story by _ Susan Allen





